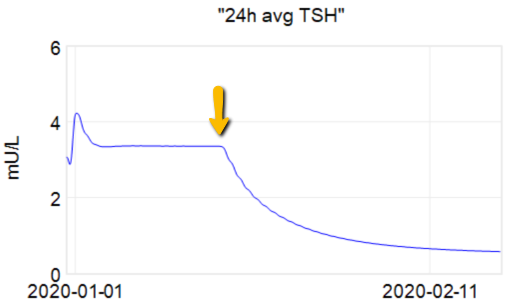
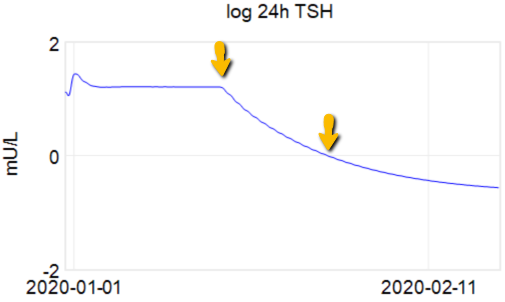
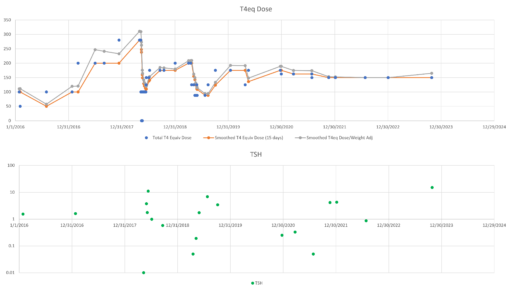
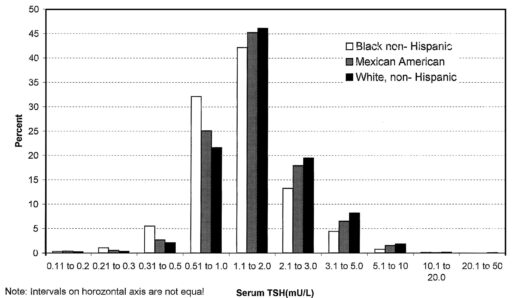
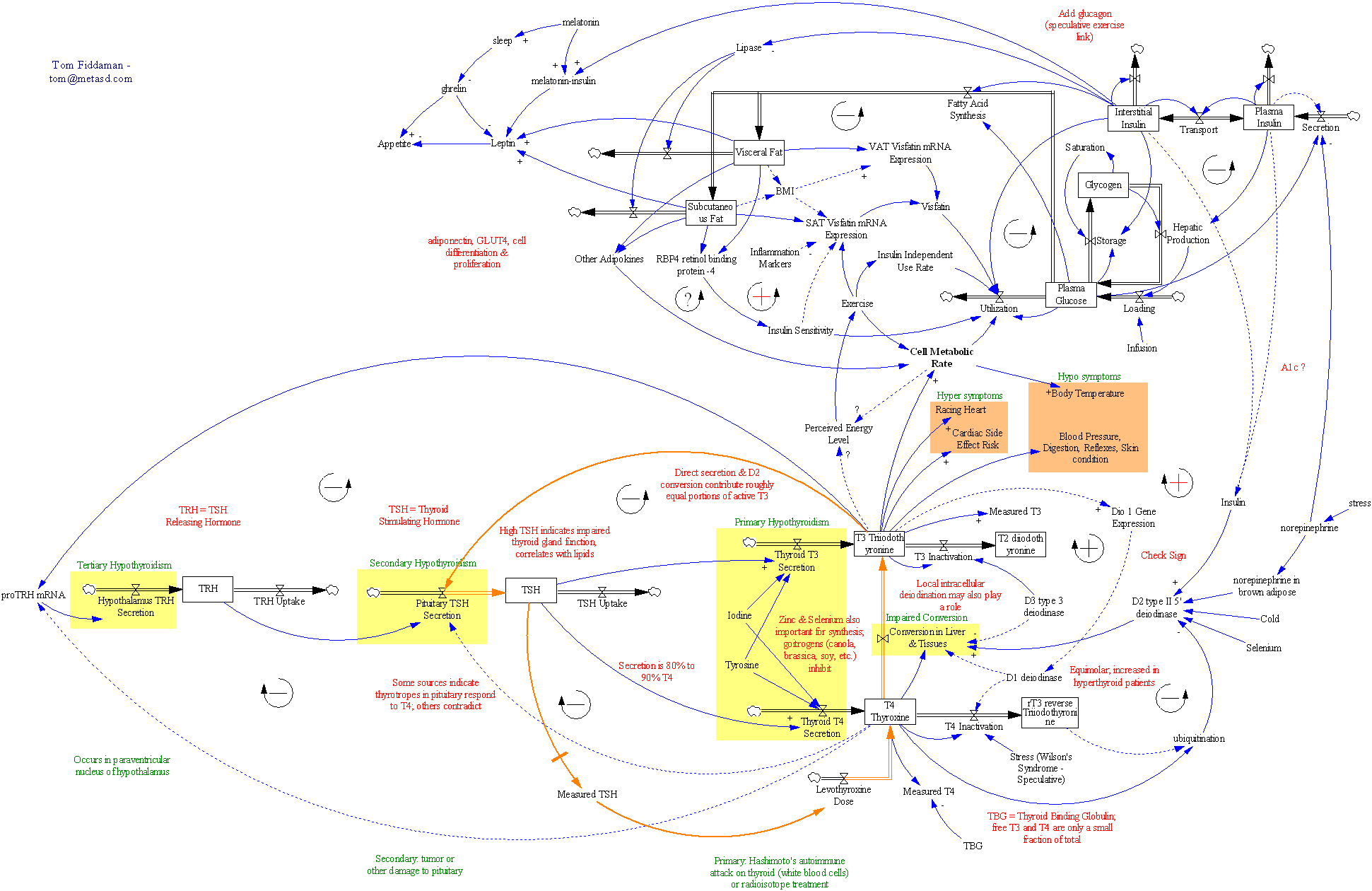
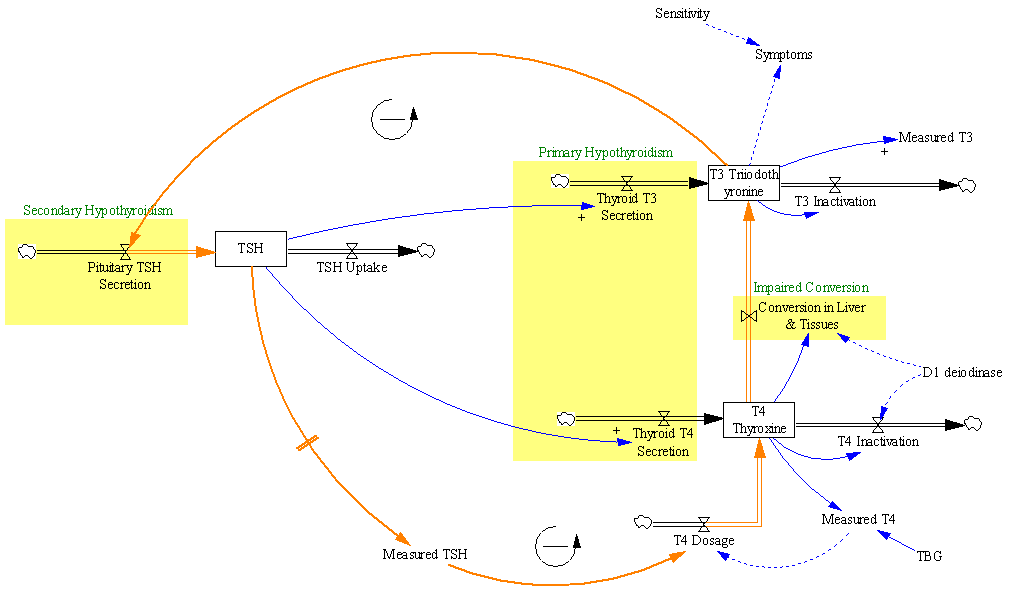
In my last thyroid post, I described a classic case of overshoot due to failure to account for delays. I forgot to mention the trigger for this episode.
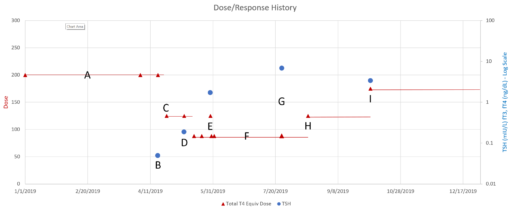
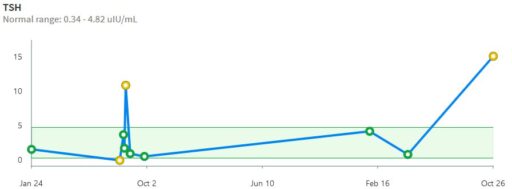
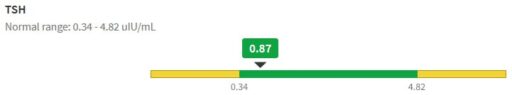
At point B above, there was a low TSH measurement, at .05 well below the recommended floor of .4. That was taken as a signal for a dose reduction, which is qualitatively reasonable.
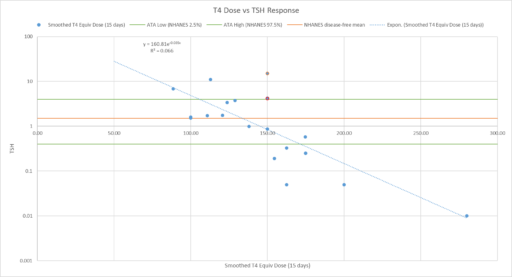
Let’s suppose we believe the dose-TSH response to be stable:
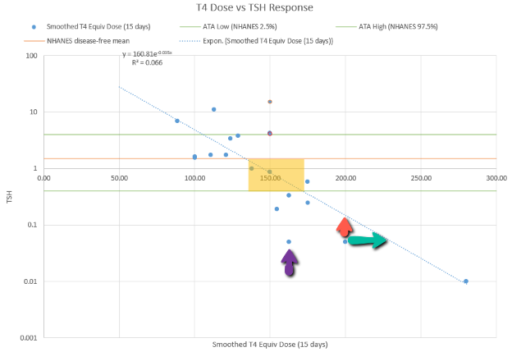 Then we have some puzzles. First, at 200mcg, we’d expect TSH=.15, about 3x higher. To get the observed measurement, we’d expect the dose to be more like 225mcg. Second, exactly the same reading of .05 has been observed at a much lower dose (162.5mcg), which is in the sweet spot (yellow box) we should be targeting. Third, also within that sweet spot, at 150mcg, we’ve seen TSH as high as 15 – far out of range in the opposite direction.
Then we have some puzzles. First, at 200mcg, we’d expect TSH=.15, about 3x higher. To get the observed measurement, we’d expect the dose to be more like 225mcg. Second, exactly the same reading of .05 has been observed at a much lower dose (162.5mcg), which is in the sweet spot (yellow box) we should be targeting. Third, also within that sweet spot, at 150mcg, we’ve seen TSH as high as 15 – far out of range in the opposite direction.
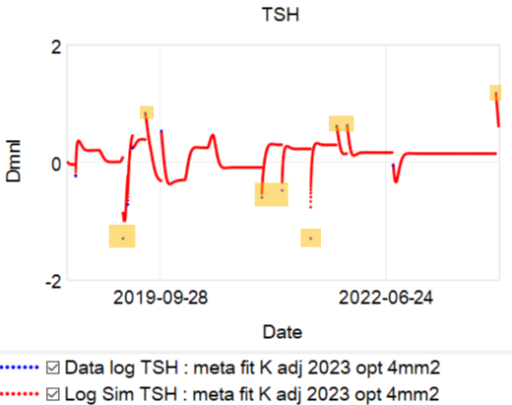
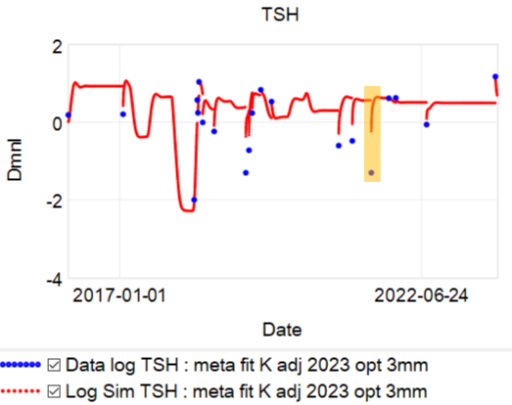
I think an obvious conclusion is that noise in the system is extreme, so there’s good reason to respond by discounting the measurement and retesting. But that’s not what happens in general. Here’s a plot of TSH observations (x, log scale) against subsequent dose adjustments (y, %):
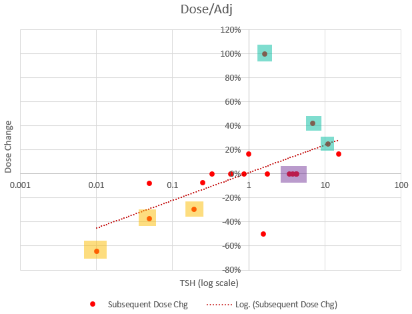 There are three clusters of note.
There are three clusters of note.
- The yellow-highlighted points are low TSH values that were followed by large dose reductions, exceeding guidelines.
- The green points are large dose increases needed to restore the yellow changes, when they subsequently proved to be errors.
- The purple points (3 total) are high TSH readings, right at the top of the recommended range, that did not induce a dose increase, even though they were accompanied by symptom complaints.
This is interesting, because the trendline seems to indicate a reasonable, if noisy, strategy of targeting TSH=1. But the operative decision rule for several of the doctors involved seems to be more like:
- If you get a TSH measurement at the high end of the range, indicating a dose increase might be appropriate, ignore it.
- If you get a low TSH measurement, PANIC. Cut dose drastically.
- If you’re the doctor replacing the one just fired for screwing this up, restore the status quo.
Why is this? I think it’s an error in reasoning. Low TSH could be caused by excessive T4 levels, which could arise from (a) overtreatment of a hypothyroid patient, or (b) hyperthyroid activity in a previously hypothyroid patient. In the case described previously, evidence from T4 testing as well as the long term relationship suggested that the dose was 20-30% high, but it was ultimately reduced by 60%. But in two other cases, there was no T4 confirmation, and the dose was right in the middle of its apparent sweet spot. That rules out overtreatment, so the mental model behind a dose reduction has to be (b). But that makes no sense. It’s a negative feedback system, yet somehow the thyroid has increased its activity, in response to a reduction in the hormone that normally signals it to do so? Admittedly, there are possibilities like cancer that could explain such behavior, but no one has ever explored that possibility in N=1’s case.
I think the basic problem here is that it’s hard to keep a mechanistic model of a complex hormone signalling system in your head, which makes it easy to get fooled by delays, feedback, noise and nonlinearity. Bad information systems and TSH monomania contribute to the problem, as does ignoring dose guidelines due to overconfidence.
So what should happen in response to a low TSH measurement in patient N=1? I think it’s more like the following:
- Don’t panic.
- It might be a bad measurement (labs don’t correct for seasonality, time of day, and other features that could inflate variance beyond the precision of the test itself).
- It might be some unknown source of variability driving TSH, like food, medications, or endogenous variation in upstream hormones.
- Look at the measurement in context of other information: the past dose-response relationship, T4, and symptoms, and reference dose per unit body mass.
- Make at most a small move, wait for the guideline-prescribed period, and retest.